Process Development and Scale Up for Adherent Cell Culture
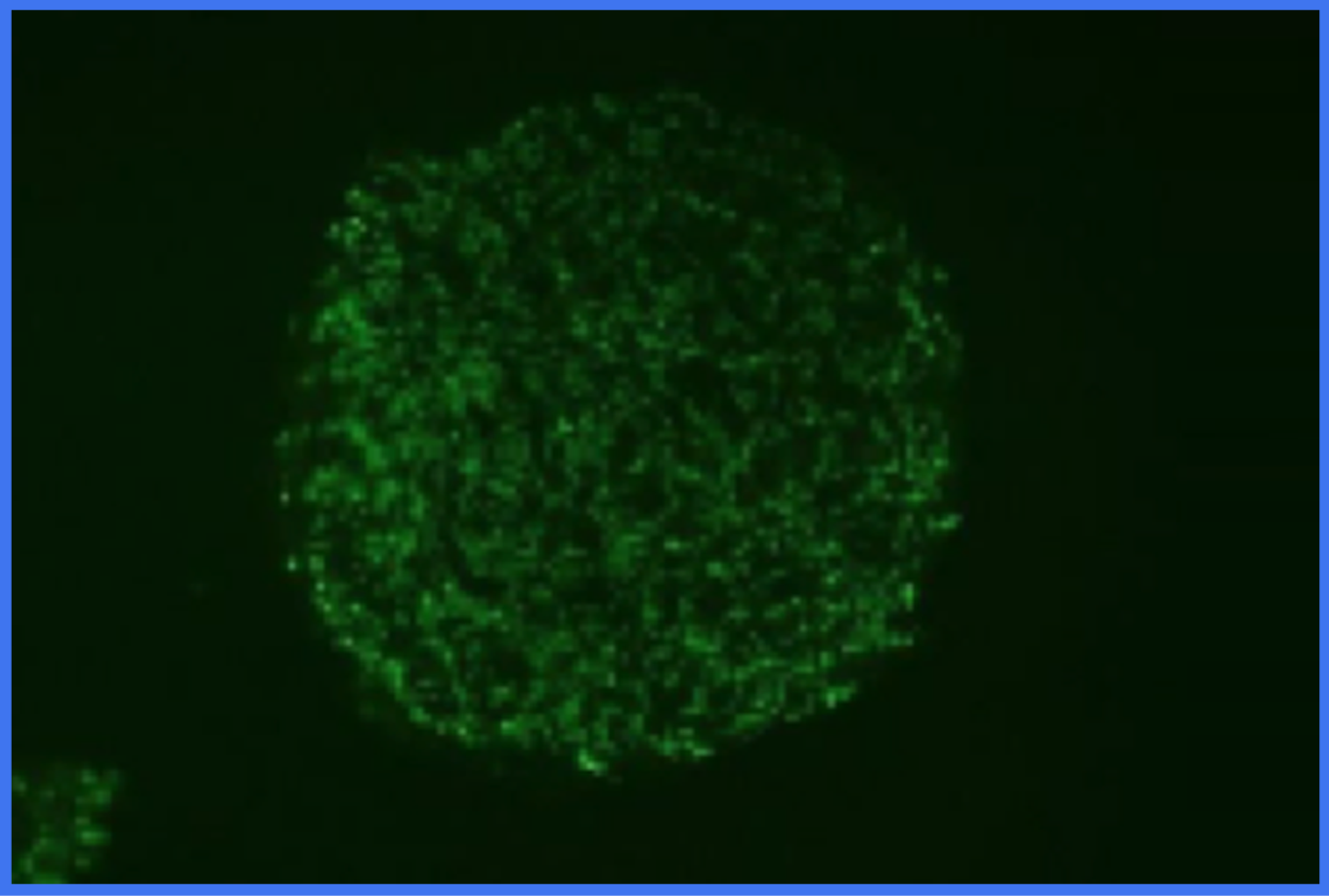
Viable Cells (stained green) immobilized in a porous scaffold.

Years of Experience
Cytocarrier Systems (CCS) Ltd has 40 years of experience in process and bioreactor development in cell based bioprocessing
Processes include:
Monoclonal Antibodies, Recombinant proteins, Viral vectors, Viral Vaccines, Stem cells and Microtissues (organoids)
Bioreactor Systems (Adherent and Suspension) include:
Stirred tank (Suspension and/or Microcarriers), fixed bed, and fluidised bed in batch, fed batch and perfusion mode
Adherent Cell Culture
Adherent cell-based bioprocessing.
- The Biopharmaceutical industry has been almost entirely based on suspension adapted CHO cell lines in the last 30+ years.
- There is a lack of bioreactors at a sufficient scale for commercial production
- There has been minimal investment in adherent cell bioreactor technology due to lack of demand in last 30 years.
- There has been a loss of expertise in large scale adherent cell culture and systems.
- There is a shortage of scalable high density perfusion bioreactors for adherent cells due to company closures in the 90’s.
- There is a lack of demand from the vaccine industry due to over-reliance on old production technology
This situation is changing due to the commercialization of Cell and Gene Therapy Therapeutics.
- Some stem cells are strictly adherent, e.g. Mesenchymal Stromal Cells (MSC).
- Some cells need to be grown in 3D to mimic the tissue of origin to retain their function.
- Many novel viral vectors and vaccines require adherent cells to grow sufficiently despite the push from industry to switch to suspension-based bioreactor systems
Lab Technicians
Lorem ipsum dolor sit amet, consectetur adipiscing elit.
Research Center
Lorem ipsum dolor sit amet, consectetur adipiscing elit.
ABOUT US
In-depth expertise
We have in-depth expertise in research development and manufacturing from adherent cells.
Primary Company Focus
Translation of adherent cell-based culture systems from research laboratory scale into fully developed, optimised, characterised and scaled-up 2D/3D bioreactor processes.

Adherent cells growing on non-porous microcarriers
Product Development
Explore our main Products in Development

Porous Carriers for Adherent and 3D Cell Culture
Immobilised Bed Bioreactor for 3D Cell Culture in Porous Carriers
Disposable Stirred Tank Bioreactor for the Culture of Cells Immobilised in Porous Carriers
Research & Development Collaborations
Explore our Research and Development Collaborations
- Cytocarrier Systems (CCS) Ltd has significant experience in setting up, project management, and participation in European Union and Enterprise Ireland funded projects in Bioprocessing and Bioreactor Development.
- CCS Ltd is a validated SME for participating in EU projects.
- If you are considering applying for EU (Horizon), EI or SFI funding in bioprocessing, cell and Gene therapy, and/or 3D culture, we would be happy to discuss how we might enhance your project.
- CCS Ltd is setting up a number of collaborations with Biotechnology Companies and Research Organizations to test our scalable 3D cell culture systems for adherent cell and organoid cultures.
- CCS Ltd is interested in talking to any organizations who are looking to develop scalable and controllable processes for adherent cells in cell and gene therapy and viral vaccine domains
Consultancy Service
Explore our main Consultancy Services
Process
development
We can help you identify the most suitable cell culture system for your process. We can guide you through the first stages in evaluating culture systems for your cell line. We can guide you through the development and optimization of your process
Process
characterisation
We can help you establish your process characterisation study.
We can set up your experimental design and analyze your data
Process
scale up
We can guide you in the scale up calculations. Identify and test the operating parameters for your scale up experiment. Assist you in executing your scale-up runs.
How We Work
Our Working Process
01
Clients Projects
Sed ut perspiciatis unde omnis iste natus error sit voluptatem
02
General Proposal
Sed ut perspiciatis unde omnis iste natus error sit voluptatem
03
Testing Begins
Sed ut perspiciatis unde omnis iste natus error sit voluptatem
04
Reports Delivered
Sed ut perspiciatis unde omnis iste natus error sit voluptatem
LATEST Cases
Our Latest Case Studies
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua.
Our Expert
Meet Our Bioprocessing Scientist

Denis Looby
Managing Director and Founder
Testimonials
Our Collaborators
"I have worked collaboratively with Denis for around 35 years. He is an agile problem-solver, with an in-depth knowledge of the Bioprocessing Industry. His particular research based knowledge and experience has lead to his informed remedial approach to resolving development and production problems. This is valuable to start up and established companies wishing to develop novel Cell and Gene Therapy and/or Regenerative Medicine Manufacturing Processes."
Richard Fry, Cellon SA
"Denis is a knowledgeable and experienced Bioprocess Development and Production Scientist. He is adept at identifying and resolving development and production related issues. When I was site lead with Genemedix, Denis was a lead contributor to the development, characterization, validation, scale-up and commercial manufacture of a biosimilar EPO. The main upstream unit operation of which was an adherent CHO high density perfusion bioreactor process."
Conor O’Dea, Site Lead, Zoetis
"I’ve known Denis for over 20 years and have worked with him on multiple client projects since 2014.
Denis’s collaborative style and scientific approach to problem solving for process development has consistently delivered practical solutions for clients. Cyto Carrier Systems promises to deliver process innovations and technologies for adherent cell culture applications. These innovations are likely to have a significant impact on the commercialization potential of cell and gene therapy and RegenMed processes."
Dr. R. O'Kennedy, Director and Principal consultant, ROK Bioconsulting
"Denis was working for Menarini Biotech during the time I was Chief Operations Director in the company. His contribution to the success of the Process Development Group was definitive, bringing on-board new techniques and a way of working very much appreciated by everyone at the company”
Alfredo Martínez, COO, VIVEbiotech
Client
Trusted by world's companies.
SUBSCRIBE & FOLLOW US
Subscribe & Get More Information